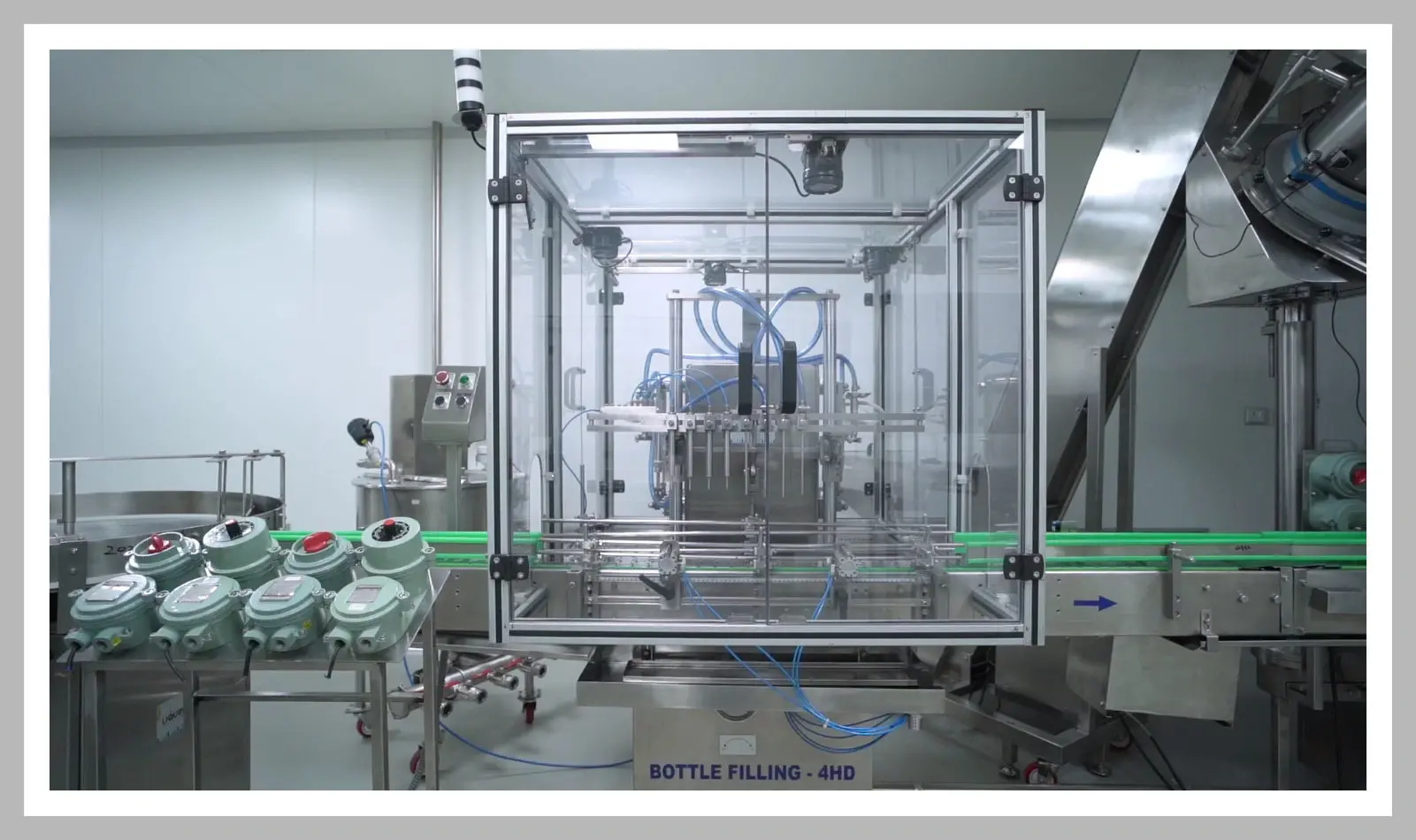
State of the art facility with advanced manufacturing techniques help the organization accommodate to global demand with high quality and affordable medicines.
The philosophy of our manufacturing excellence is to continuously improve on processes, eliminate inefficiencies and facilitate lean procedures.
Our processes are carefully automated to consistently match output demand through a well-established regulatory framework ensures that our plant can consistently deliver products of global quality standards.
All Formulation and Developmental activities are conducted as per global regulatory guidelines through Inhouse quality controlled designs using cGMP, cGLP, cGDP and Quality by Design (QbD) concept.
Our plant capacity is adequate to accommodate voluminous production schedules.
| Manufacturing Unit Details | |
|---|---|
| Facilities | Tablets, Capsules, Liquid Orals |
| Site Area | 2,08,249 sq. ft. |
| Built-up Area | 1,10,000sq. ft. |
| Production Area | 32,0000 sq. ft. |
| QC Area | 8,000 sq. ft. |
| Admin & QA Area | 8,000 sq. ft. |
| Warehouse Area | 54,000 ft3 |
| Utility Area | 7,600 sq. ft. |
The cGMP manufacturing facility at Gujarat is designed to comply with stringent USFDA and other regulatory standards of approval.
The layout for our formulation facility is as per cGMP and global regulatory guidelines. Production area is equipped for manufacturing Oral Solid Dosage forms at a scale of 150 kg in a single lot. From dedicated Air Handling Units (AHU) with air locks and differential pressures for each operation along different areas, Compression machines, automated Coating machines, Alu-Alu blister packing machines to automated bottle filling lines, our products are manufactured using the most upgraded technology adhering to cGMP and global regulatory guidelines.